Data & Protocols
Data
Individuals can access public SCORCH data on the SCORCH data portal. Restricted data will be available through dbGaP.
Protocols
Broad SCORCH
Mt. Sinai SCORCH (Human)
Y-SCORCH
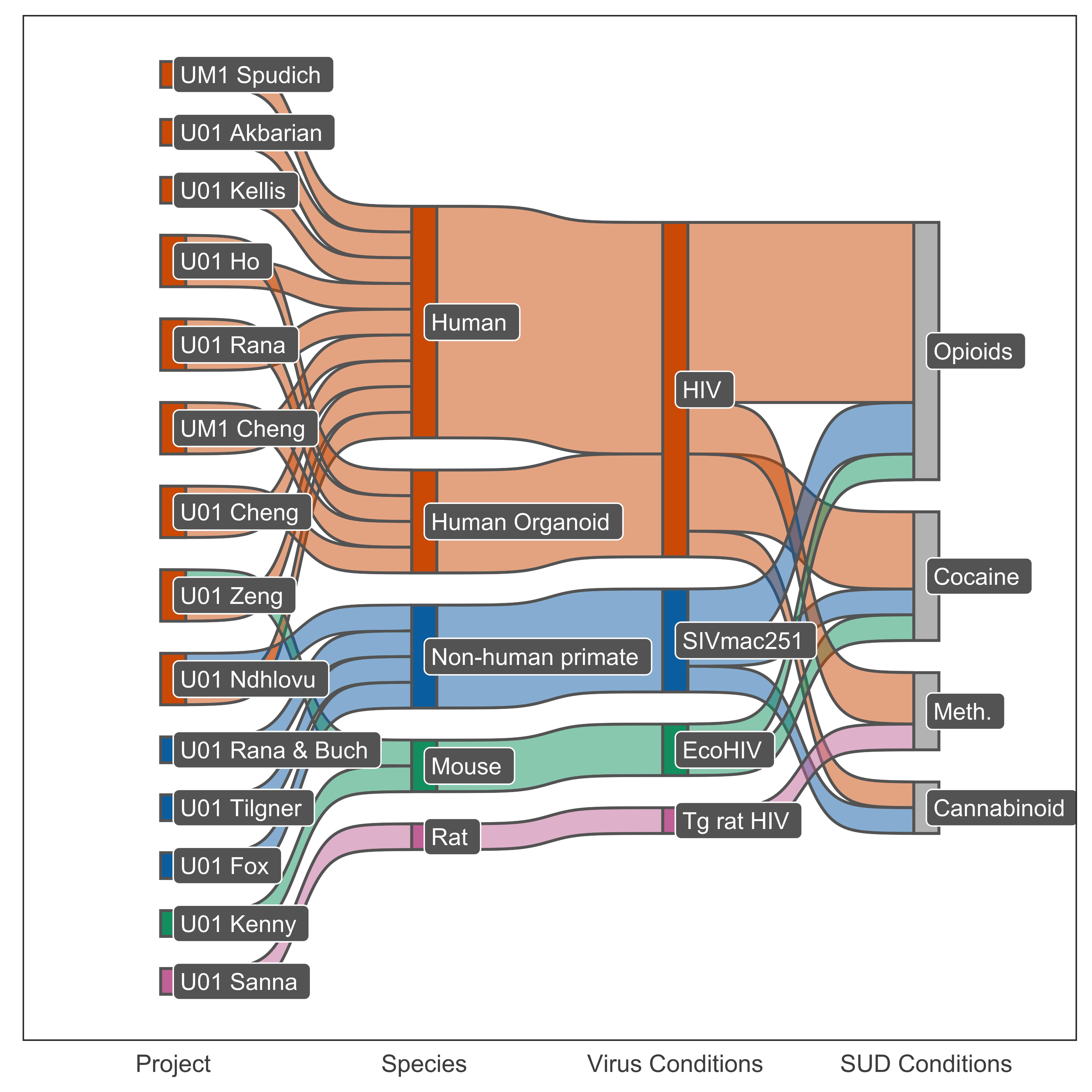
Fifteen data generation projects are currently funded by the SCORCH initiative:
UCSD SCORCH (Cheng, 5UM1DA051411-03)
This project will perform single nucleus RNA sequencing (snRNA-seq) on three brain regions with the possibility of identifying gene targets that exhibit region and cell type specific selectivity of opioid use disorder in the context of HIV/HIV associated neurocognitive impairment.
Principal Investigators
- Christine S. Cheng, PhD
csc003@health.ucsd.edu
University of California San Diego
UCSD SCORCH (Cheng, 1U01DA056006-01)
This study will characterize single nuclei gene expression and identify dysregulated gene regulatory networks in each of the neuronal and glial populations associated with cocaine misuse in HIV infected individuals and/or with HANDs. We will also perform computational analysis to identify neuronal and glial cell regulatory networks altered by cocaine misuse. In the validation and functional characterization component, we will characterize top genes in 3D brain organoid model and will characterize with CRISPR knockout and overexpression of the gene.
Principal Investigators
- Christine S. Cheng, PhD
csc003@health.ucsd.edu
University of California San Diego - David J. Moore, PhD
cdjmoore@health.ucsd.edu
University of California San Diego - Stephen A. Spector, PhD
saspector@health.ucsd.edu
University of California San Diego
UCSD SCORCH (Rana, 1U01DA053630-01)
This project puts forward an unprecedented concept and experimental system to identify single cell determinants of the brain relevant to persistent HIV infection and opioid use disorder as well as potential opportunities to illuminate how genetic variation affects gene expression.
Principal Investigators
- Tariq Rana, PhD
trana@health.ucsd.edu
University of California San Diego
UCSD SCORCH (Rana, 1U01DA058402-01)
The overall objective of this project is to reveal the single cell determinants of brain in the context of viral persistence in SIV/cART/cocaine non-human primates. Simultaneous single-cell RNA sequencing and single-cell Assay for Transposase- Accessible Chromatin sequencing (scMultiome-seq) will be carried out on cells isolated from the prefrontal cortex (PFC), striatum, and hippocampus from each animal. Data will be processed and analyzed to derive cell type-specific regulatory programs and changes in these programs that regulate neuropathogenesis in the primate brains. These studies will provide unique and valuable insights into SIV/cocaine/cART interactions in the brain while also providing crucial experimental validation of the findings in humans.
Principal Investigators
- Tariq Rana, PhD
trana@health.ucsd.edu
University of California San Diego - Shilpa Buch, PhD
sbuch@unmc.edu
University of Nebraska Medical Center
Y-SCORCH (UM1DA051410-01)
This project will perform snRNA-seq, single-nucleus ATAC-seq (scATAC-seq), and spatial transcriptomics on 20 brains from four donor groups: controls, HIV (HIV+), HIV with OUD (HIV+OUD+), and OUD without HIV (OUD+). Three regions which represent disease-relevant areas for OUD and HIV, will be assayed: prefrontal cortex, ventral striatum and insular cortex.
Principal Investigators
- Serena Spudich, MD
serena.spudich@yale.edu
Yale School of Medicine
Department of Neurology - Mark Gerstein, PhD
mark@gersteinlab.org
Yale School of Medicine - Yuval Kluger, PhD
yuval.kluger@yale.edu
Yale School of Medicine
M-SCORCH (U01DA053628-01)
This project will generate a single-cell transcriptome and regulome atlas of gene regulatory networks in the human brain during neuroimmunologic perturbations by methamphetamine and HIV infection.
Principal Investigators
- Ya-Chi Ho, MD, PhD, MMS
ya-chi.ho@yale.edu
Yale School of Medicine - Nenad Sestan, MD, PhD
nenad.sestan@yale.edu
Yale School of Medicine
Mount Sinai SCORCH (Akbarian, U01DA053600-01)
This project will engage in mapping cell specific transcriptomes in monoaminergic circuitry associated with prospectively monitored neurological status in the years before death and exposure to drug of abuse. It will also explore whether or not opiate and cocaine exposures affect HIV neurogenomic activity.
Principal Investigators
- Schahram Akbarian, MD, PhD
schahram.akbarian@mssm.edu
Icahn School of Medicine at Mount Sinai - Susan Morgello, MD
susan.morgello@mssm.edu
Icahn School of Medicine at Mount Sinai
Mount Sinai SCORCH (Kenny, U01DA053629-01)
This project will leverage state-of-the-art single cell and single nuclei RNA sequencing combined with molecular, cellular and behavioral approaches to define cell type-specific interactions between HIV and opioids in the brains of EcoHIV-infected mice.
Principal Investigators
- Paul Kenny, PhD
paul.kenny@mssm.edu
Icahn School of Medicine at Mount Sinai - David Volsky, PhD
david.volsky@mssm.edu
Icahn School of Medicine at Mount Sinai
UNMC SCORCH (1U01DA053624-01)
This project will perform studies on the interplay of HIV pathogenesis and opioid abuse in the gold-standard SIV/nonhuman primate system, using single-cell RNA sequencing (scRNAseq) to assess individual cellular transcriptomes.
Principal Investigators
- Howard Fox, MD, PhD
hfox@unmc.edu
University of Nebraska Medical Center - Shilpa Buch, PhD
sbuch@unmc.edu
University of Nebraska Medical Center
BROAD SCORCH (1U01DA053631-01)
This project will dissect the transcriptional and epigenetic changes associated with SUD and persistent HIV infection at single-cell resolution across seven human brain regions, and integrate the resulting datasets to predict driver genes.
Principal Investigators
- Manolis Kellis, PhD
manoli@mit.edu
The Broad Institute - Myriam Heiman, PhD
mheiman@mit.edu
The Broad Institute
Weill Cornell SCORCH (Tilgner, 1U01DA053625-01)
This projects seeks to generate topographical data sets and evidence at single cell resolution across the hippocampus and prefrontal cortex (PFC), two brain regions known for predilection for HIV persistence and OUD in non-human primate (NHP) and in post-mortem human brain. These data will provide an unprecedented cellular landscape of multiple modalities that can be harnessed to develop strategies to limit viral persistence and restore and retain optimal brain health in people living with HIV.
Principal Investigators
- Hagen Tilgner, PhD
hut2006@med.cornell.edu
Weill Cornell Medicine - Teresa Milner, PhD
tmilner@med.cornell.edu
Weill Cornell Medicine - Lishomwa Ndhlovu, MD, PhD
lndhlovu@med.cornell.edu
Weill Cornell Medicine
Weill Cornell SCORCH (Ndhlovu, 1U01DA058527-01)
To identify cell types, epigenetic cell states, and gene pathways relevant to neuropathogenesis, viral persistence, and cannabinoid exposures, we are harnessing 180 brain tissue samples from an established oral administration of either cannabidiol (CBD) or Δ9- tetrahydrocannabinol (THC) in NHP and single cell assays (10X Genomics single nucleus multiome and a new single cell assay developed at the NYGC capable of measuring the genome-wide presence of multiple histone modifications and protein-DNA binding sites). Moreover, accompanying single cell data will be generated from conserved neurogenic brain regions of human postmortem brain tissues from 40 donors based on HIV status (+/-) and cannabis exposure (+/-). We will also explore the frequency of single cells in neurogenic regions of the brain that are infected and impacted by cannabinoids by harnessing a bioinformatics pipeline that detects both viral transcripts and transposase accessible provirus. This project will generate comprehensive single cell datasets in NHP and humans to improve our understanding of the cross talk between HIV and cannabinoids in neurogenic regions of the brain and has high programmatic priority to goals of the SCORCH program expansion.
Principal Investigators
- Lishomwa Ndhlovu, MD, PhD
lndhlovu@med.cornell.edu
Weill Cornell Medicine - Michael J. Corley, PhD
mjc4002@med.cornell.edu
Weill Cornell Medicine - Dionna Whitney Williams, PhD
dwill201@jhmi.edu
Johns Hopkins University
Scripps Research SCORCH (Sanna, 1U01DA056004-01)
The abuse of stimulants such as methamphetamine (METH) exacerbates the deleterious effects of HIV infection. This project will carry out single nucleus RNA-Seq with the goal of identifying cell types and cell states that are pivotal in the effects of HIV and chronic methamphetamine (METH) self-administration on key brain regions relevant to the effects of persistent HIV infection and METH use disorder in HIV transgenic (Tg) rats, which harbor a non-replicating HIV-1 transgene and express chronic low-levels of multiple HIV-1 proteins.
Principal Investigators
- Pietro Sanna, MD
psanna@scripps.edu
Scripps Research
Scripps Research SCORCH (Sanna, 5U01DA056004-02)
This project will carry out single nucleus RNA-Seq with the goal of identifying cell types and cell states that are pivotal in the effects of HIV and chronic methamphetamine (METH) self-administration on key brain regions relevant to the effects of persistent HIV infection and METH use disorder in HIV transgenic (Tg) rats, which harbor a non-replicating HIV-1 transgene and express chronic low-levels of multiple HIV-1 proteins. We expect this project to identify novel mechanistic hypotheses that may lead to transformative new therapeutic concepts for substance use disorder (SUD) in the HIV setting, and will establish key resources for the neuroHIV field to be made publicly available through the SCORCH data coordination center.
Principal Investigators
- Pietro Sanna, MD
psanna@scripps.edu
Scripps Research
Allen Institute SCORCH (1U01DA056003-01)
This project will leverage state-of-the-art single-nuclei RNA sequencing (snRNA-seq) coupled to single-nuclei epigenetics (snATAC-seq) to define the transcriptional and epigenetic landscape of neuronal and nonneuronal cells in addiction-relevant brain cells of HIV-infected mice that compulsively self-administer cocaine. In this manner cell types and brain sites in which HIV and cocaine interact to exacerbate the negative impact of HIV infection on the brain and contribute to the persistence of cocaine use disorder in infected individuals will be identified.
Principal Investigators
- Hongkui Zeng, PhD
HongkuiZ@alleninstitute.org
Allen Institute - Paul Kenny, PhD
paul.kenny@mssm.edu
Icahn School of Medicine at Mount Sinai - David Volsky, PhD
david.volsky@mssm.edu
Icahn School of Medicine at Mount Sinai









